Nori Equine Calprotectin ELISA Kit
$461.00 – $832.00
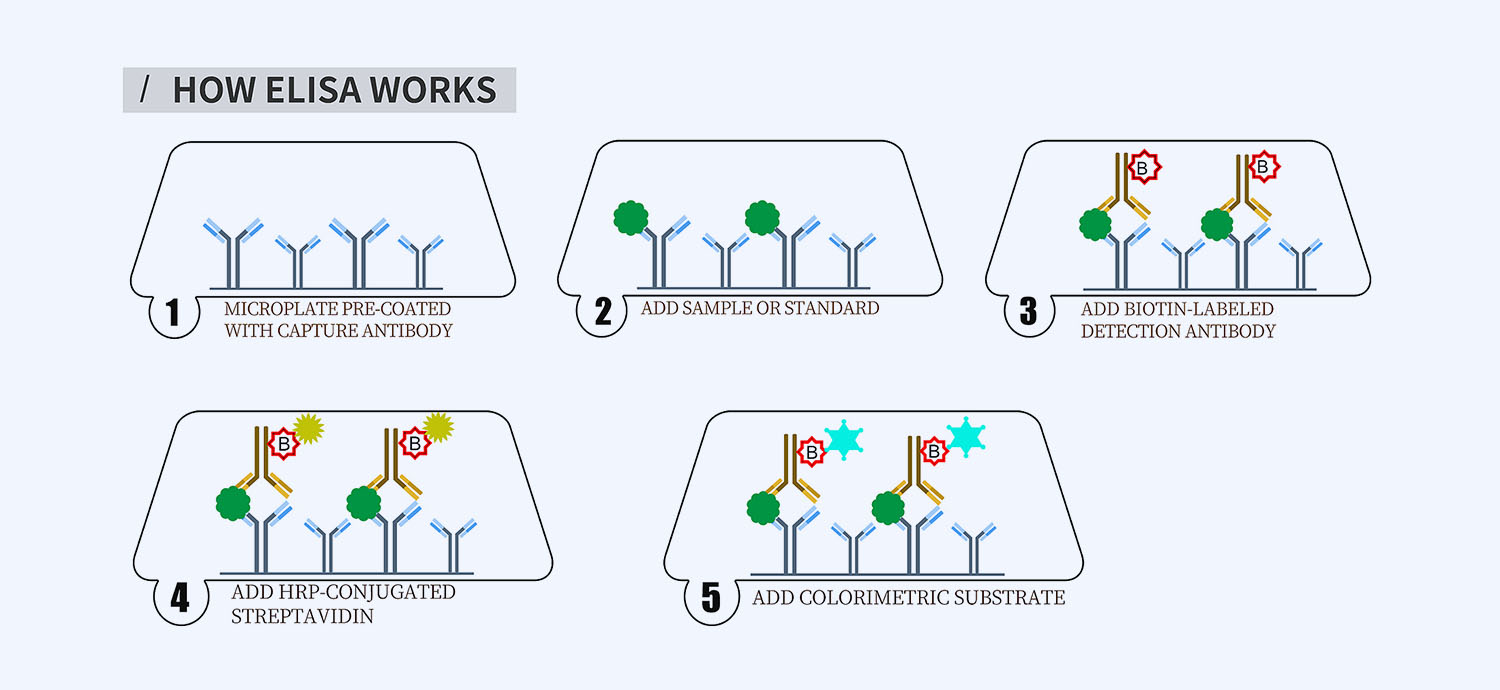
This ELISA kit is for quantification of calprotectin in horse. This is a quick ELISA assay that reduces time to 50% compared to the conventional method, and the entire assay only takes 3 hours. This assay employs the quantitative sandwich enzyme immunoassay technique and uses biotin-streptavidin chemistry to improve the performance of the assays. An antibody specific for calprotectin has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any calprotectin present is bound by the immobilized antibody. After washing away any unbound substances, a detection antibody specific for calprotectin is added to the wells. Following wash to remove any unbound antibody reagent, a detection reagent is added. After intensive wash a substrate solution is added to the wells and color develops in proportion to the amount of calprotectin bound in the initial step. The color development is stopped, and the intensity of the color is measured.
Alternative names for calprotectin: dimer of calcium binding proteins S100A8 and S100A9
This product is for laboratory research use only not for diagnostic and therapeutic purposes or any other purposes.
- Description
- How Elisa Works
- Product Citation
- Reviews (0)
Description
Nori Equine Calprotectin ELISA Kit Summary
Alternative names for calprotectin: dimer of calcium binding proteins S100A8 and S100A9
Alternative names for equine: Horse
| Assay Type | Solid Phase Sandwich ELISA |
| Format | 96-well Microplate or 96-Well Strip Microplate |
| Method of Detection | Colorimetric |
| Number of Targets Detected | 1 |
| Target Antigen Accession Number | na |
| Assay Length | 3 hours |
| Quantitative/Semiquantitative | Quantitative |
| Sample Type | Plasma, Serum, Cell Culture, Urine, Cell/Tissue Lysates, Synovial Fluid, BAL, |
| Recommended Sample Dilution (Plasma/Serum) | No dilution for sample <ULOQ; sufficient dilution for samples >ULOQ |
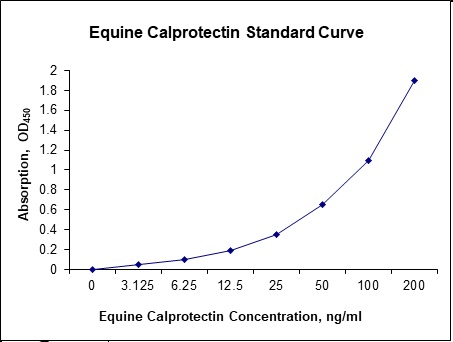
| Sensitivity | 600 pg/mL |
| Detection Range | 3.125-200 ng/mL |
| Specificity | Natural and recombinant equine calprotectin |
| Cross-Reactivity | < 0.5% cross-reactivity observed with available related molecules, < 50% cross-species reactivity observed with species tested. |
| Interference | No significant interference observed with available related molecules |
| Storage/Stability | 4 ºC for up to 6 months |
| Usage | For Laboratory Research Use Only. Not for diagnostic or therapeutic use. |
| Additional Notes | The kit allows for use in multiple experiments. |
Standard Curve
Kit Components
1. Pre-coated 96-well Microplate
2. Biotinylated Detection Antibody
3. Streptavidin-HRP Conjugate
4. Lyophilized Standards
5. TMB One-Step Substrate
6. Stop Solution
7. 20 x PBS
8. Assay Buffer
Other Materials Required but not Provided:
1. Microplate Reader capable of measuring absorption at 450 nm
2. Log-log graph paper or computer and software for ELISA data analysis
3. Precision pipettes (1-1000 µl)
4. Multi-channel pipettes (300 µl)
5. Distilled or deionized water
Protocol Outline
1. Prepare all reagents, samples and standards as instructed in the datasheet.
2. Add 100 µl of Standard or samples to each well and incubate 1 h at RT.
3. Add 100 µl of Working Detection Antibody to each well and incubate 1 h at RT.
4. Add 100 µl of Working Streptavidin-HRP to each well and incubate 20 min at RT.
5. Add 100 µl of Substrate to each well and incubate 5-30 min at RT.
6. Add 50 µl of Stop Solution to each well and read at 450 nm immediately.
Background:
Calprotectin is a 24 kDa dimer of calcium binding proteins S100A8 and S100A9.[1] The complex accounts for up to 60% of the soluble protein content of the neutrophil cytosol. In vitro studies show that calprotectin has bacteriostatic and fungistatic properties, that arise from its ability to sequester manganese and zinc.[1] Calprotectin has two transition metal binding sites that form at the interface of the S100A8 and S100A9 monomers, and metal sequestration by calprotectin has been shown to be calcium dependent.[1] The complex is resistant to enzymatic degradation, and can be easily measured in faeces.[2] Fecal calprotectin is a biochemical measurement of the protein calprotectin in the stool. Elevated faecal calprotectin indicates the migration of neutrophils to the intestinal mucosa, which occurs during intestinal inflammation, including inflammation caused by inflammatory bowel disease. Under a specific clinical scenario, the test may eliminate the need for invasive colonoscopy or radio-labelled white cell scanning. The main diseases that cause an increased excretion of faecal calprotectin are inflammatory bowel diseases, coeliac disease, infectious colitis, necrotizing enterocolitis, intestinal cystic fibrosis and colorectal cancer.[3] Although a relatively new test, faecal calprotectin is regularly used as indicator for inflammatory bowel diseases (IBD) during treatment and as diagnostic marker. IBD are a group of conditions that cause a pathological inflammation of the bowel wall. Crohn’s disease and ulcerative colitis are the principal types of inflammatory bowel disease. Inflammatory processes result in an influx of neutrophils into the bowel lumen.[4] Since calprotectin comprises as much as 60% of the soluble protein content of the cytosol of neutrophils, it can serve as a marker for the level of intestinal inflammation.[5] Measurement of faecal calprotectin is considered the gold standard measurement of intestinal inflammation.[6] Levels of faecal calprotectin are usually normal in patients with irritable bowel syndrome (IBS).[7] In untreated coeliac disease, concentrations of faecal calprotectin correlate with the degree of intestinal mucosal lesion and normalize with a gluten-free diet. Although faecal calprotectin correlates significantly with disease activity with confirmed IBD, faecal calprotectin can be false-positive if the laboratory uses low calprotectin cut-off levels.[7] Most importantly, intake of non-steroidal anti-inflammatory drugs (aspirin included) increases calprotectin values, possibly due to the associated induced enteropathy.
References
- Brophy MB, Nolan EM (2015). ACS Chemical Biology. 10(3): 641–51.
- Tibble J, et al. (2000). Gut. 47(4): 506–13.
- Konikoff MR, et al. (2006). Inflammatory Bowel Diseases (Review). 12(6): 524–34.
- Walsham NE, et al. (2016). Clinical and Experimental Gastroenterology. 9: 21–9.
- Stríz I, Trebichavský I (2004). Physiological Research. 53(3): 245–53.
- Costa F, et al. (2003). Digestive and Liver Disease. 35(9): 642–7.
- Waugh N, et al. (2013) Health Technology Assessment (Review) 17(55): xv–xix, 1–211.
Product Citation
Be the first to review “Nori Equine Calprotectin ELISA Kit”
You must be logged in to post a review.






















Reviews
There are no reviews yet.